CLINICAL
EVIDENCE
EVALUATION OF ACTIPATCH® IN A LANDMARK 5000 CHRONIC PAIN PATIENT STUDY
HOW THE STUDY WORKED:
RESULTS:
ActiPatch® reduced pain by more than 50% in two-thirds of patients after the 7-day trial and relief was sustained for more than 3 months with regular use.2
No major adverse events were reported.2
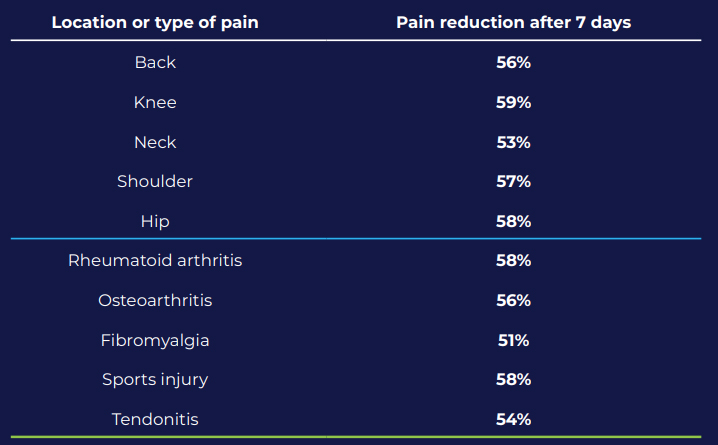
CONCLUSION:
ActiPatch® appears to provide a clinically meaningful reduction of chronic muscle and joint pain affecting different locations of the body caused by a variety of conditions.2 ActiPatch® offers a new alternative chronic pain therapy.2
EVALUATION OF ACTIPATCH® IN A 6-MONTH CHRONIC PAIN PATIENT STUDY
HOW THE STUDY WORKED:
RESULTS:
THE BENEFITS OF ACTIPATCH®
EXTENDED BEYOND PAIN RELIEF

CONCLUSION:
Regular use of ActiPatch® sustained pain relief for 6 months in over 85% of chronic pain patients, and substantially improved physical activity, sleep quality, and overall quality of life.3 A decreased consumption of pain medication, including prescription and opioid-based pain medications.3
EVALUATION OF ACTIPATCH® IN KNEE OSTEOARTHRITIS
HOW THE STUDY WORKED:
RESULTS:

CONCLUSION:
ActiPatch® is effective for pain caused by osteoarthritis of the knee.7
EVALUATION OF ACTIPATCH® IN NECK OSTEOARTHRITIS
HOW THE STUDY WORKED:
RESULTS:
CONCLUSION:
In patients with neck osteoarthritis, ActiPatch® showed better pain relief with no adverse effects compared to etoricoxib.6
EVALUATION OF ACTIPATCH® IN CHRONIC BACK PAIN
HOW THE STUDY WORKED:
RESULTS:
CONCLUSION:
ActiPatch® effectively reduces chronic back pain for most patients and reduces the use of pain medication.8
EVALUATION OF ACTIPATCH® IN PLANTAR FASCIITIS
HOW THE STUDY WORKED:
RESULTS:
CONCLUSION:
ActiPatch® offers a simple, drug-free, non-invasive therapy to reduce the pain associated with plantar fasciitis.9
ActiPatch® Medical Device. ActiPatch® is a registered trademark of BioElectronics Corporation. For full prescribing information refer to the Instructions for Use. Tel: 1-866-757-2284. Marketed by Adcock Ingram Limited. Co. Reg. No. 1949/034385/06. Private Bag X69, Bryanston, 2021. Customer Care: 0860 ADCOCK/232625. www.adcock.co.za. 2023031510265247 March 2023.

© 2022 Adcock Ingram. All rights reserved
